Media
HKUMed develops a novel therapeutic approach against Epstein-Barr virus-associated tumours by using exosomes derived from Vδ2-T cells
08 Oct 2020
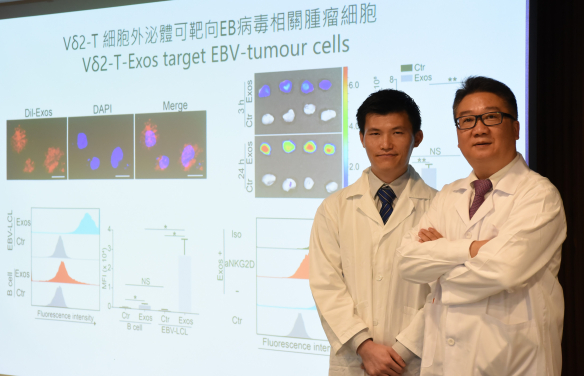
HKUMed develops a novel therapeutic approach against Epstein-Barr virus-associated tumours by using exosomes derived from Vδ2-T cells. The research was led by Professor Tu Wen-wei, Antony and Nina Chan Professor in Paediatric Immunology, Department of Paediatrics and Adolescent Medicine, HKUMed (right) and Dr Wang Xi-wei, post-doctoral fellow of Professor Tu’s team (left), is the first author.
A research team at LKS Faculty of Medicine, The University of Hong Kong (HKUMed) discovered that exosomes derived from Vδ2-T cells (Vδ2-T-Exos) can effectively control Epstein-Barr virus-associated tumours and induce T-cell anti-tumour immunity. The novel findings of Vδ2-T-Exos provide insights into new therapeutic approach for Epstein-Barr virus (EBV)-associated tumours. The ground-breaking findings have been published in the leading academic journal, Science Translational Medicine. [Link to the publication]
Background
EBV infects about 95% of the human population and causes more than 200,000 cases of cancer each year and that around 2% of all cancer deaths are due to EBV-attributable malignancies. EBV-associated tumours include Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, gastric tumour and post-transplant lymphoproliferative disease, etc. Current treatment options for EBV-associated tumours are limited with considerably unwanted off-target toxicities and incomplete effectiveness for relapsed or refractory disease. Vδ2-T cells are innate-like T cells with anti-tumour potentials against EBV-associated tumours. Unfortunately, its clinical translation is limited because Vδ2-T cells from some cancer patients are difficult to be expanded. Exosomes are endosome-originated small extracellular vesicles that mediate intercellular communication. Compared with cell-based therapy, cell-free exosomes have advantages with higher safety, easier storage, and lower costs. However, the anti-tumour activity of exosomes derived from Vδ2-T cells (Vδ2-T-Exos) remains unknown.
Research findings
Herein, the team found that Vδ2-T-Exos contained death-inducing ligands (FasL and TRAIL) and immunostimulatory molecules (CD80, CD86, MHC class I and II). Vδ2-T-Exos targeted and efficiently killed EBV-associated tumour cells through FasL and TRAIL pathways and promoted EBV antigen-specific CD4 and CD8 T cell expansion. Administration of Vδ2-T-Exos effectively controlled EBV-associated tumours in immunodeficient and humanized mice. Because expanding Vδ2-T cells and preparing autologous Vδ2-T-Exos from cancer patients ex vivo in large scale is challenging, the team further explored the anti-tumour activity of allogeneic Vδ2-T-Exos in humanized mouse cancer models. Interestingly, the team found that allogeneic Vδ2-T-Exos had more effective anti-tumour activity than autologous Vδ2-T-Exos in humanized mice; the allogeneic Vδ2-T-Exos increased the infiltration of T cells into tumour tissues and induced more robust CD4 and CD8 T cells-mediated anti-tumour immunity. Compared with exosomes derived from NK cells with direct cytotoxic anti-tumour activity or dendritic cells that induced T-cell anti-tumour responses, Vδ2-T-Exos have dual anti-tumour activities by directly killing tumour cells and indirectly inducing T cells-mediated anti-tumour responses, thus resulting in more effective control of EBV-associated tumours.
“Our study provides the first evidence about the anti-tumour activities of Vδ2-T-Exos against EBV-associated tumours. These exosomes could effectively control EBV-associated cancers in multiple mouse models. More importantly, allogeneic Vδ2-T-Exos had higher therapeutic efficacy than autologous Vδ2-T-Exos to control EBV-associated tumours. Therefore, the Vδ2-T-Exos prepared from healthy donors can be used to treat patients with EBV-associated tumours, which is highly beneficial to the clinical application of this novel approach,” said Professor Tu Wen-wei, Antony and Nina Chan professor in Paediatric Immunology, Department of Paediatrics and Adolescent Medicine, HKUMed, who led the research.
Significance of the study
The findings of the study have significant implications in cancer immunotherapy. Firstly, the identification that Vδ2-T-Exos has potent immunostimulatory property suggests that they could be designed as a cancer vaccine by serving as immune adjuvant and delivering immunogens. Secondly, the Vδ2-T-Exos has advantages over other exosome-based therapies (e.g. NK-Exos and DC-Exos) by displaying dual anti-tumour activities and are easier in preparation. Thirdly, the results that allogeneic Vδ2-T-Exos have higher anti-tumour efficacies than autologous Vδ2-T-Exos can greatly enhance the clinical feasibility of Vδ2-T-Exos, because the preparation of allogeneic exosomes does not require personalized procedures and is easier in quality control, standardization and centralization for clinical application.
About the research team
The research was led by Professor Tu Wen-wei, Antony and Nina Chan Professor in Paediatric Immunology, Department of Paediatrics and Adolescent Medicine, HKUMed. Dr Wang Xi-wei, post-doctoral fellow of Professor Tu’s team, is the first author. Other researchers include Dr Zheng Xiang, post-doctoral fellow of Professor Tu’s team; Dr Liu Yin-ping, Research Officer in Department of Paediatrics and Adolescent Medicine, HKUMed; Ms Huang Chun-yu, PhD student of Professor Tu; Dr Pei Yu-jun, post-doctoral fellow of Professor Tu’s team; Dr Pamela Lee Pui-wah, Clinical Associate Professor, Department of Paediatrics and Adolescent Medicine and Assistant Dean (Clinical Curriculum), HKUMed; Professor Godfrey Chan Chi-fung, Tsao Yen-Chow Professor in Paediatrics and Adolescent Medicine, Head and Clinical Professor of Paediatrics and Adolescent Medicine, HKUMed; Professor Lau Yu-lung, Doris Zimmern Professor in Community Child Health and Chair Professor of Paediatrics, Department of Paediatrics and Adolescent Medicine, HKUMed; Collaborators include Dr Wang Xia, post-doctoral fellow, Department of Pathology, HKUMed; Dr Helen Zhi, Director of Biostatistics and Clinical Research Methodology Unit, School of Public Health, HKUMed; Dr Wilfred Wong Hing-sang, Senior IT Manager, Department of Paediatrics and Adolescent Medicine, HKUMed. Professor Wei Hai-ming, Institute of Immunology, University of Science and Technology of China, Hefei, Anhui, Mainland China; Professor Irene Ng Oi-lin, Loke Yew Professor in Pathology, Director of State Key Laboratory of Liver Research (HKU), Chair of Pathology, HKUMed.
Collaborating institutions contributing to the research include the Institute of Immunology, University of Science and Technology of China, Hefei, Anhui, Mainland China.
This work was supported in part by General Research Fund (17122519, 17126317, 17115015, 17121214), Theme-based Research Scheme from the Research Grants Council (Project No. T11-705/14N), and The Area of Excellence Scheme (AoE/M-06/08) supported by the University Grants Committee, Government of the Hong Kong Special Administrative Region.
Media enquiries
Please contact LKS Faculty of Medicine of The University of Hong Kong by email (medmedia@hku.hk).

